Regulatory Aspects about Verification and Validation in Medical Devices Design
Q.F. Walther Ricardo Vicente Mallma
QA Specialist & Regulatory Affairs Coordinator – Medical Devices
SCR Consulting Peru
In Peru, medical devices are regulated by the Dirección General de Medicamentos, Insumos y Drogas (DIGEMID). DIGEMID, as a line organ of the Ministry of Health, supervises all aspects related to the approval, denial, modification of the sanitary registration of medical devices, as well as the establishment of the necessary requirements for their registration. This regulation is ruled by Law No. 29459: Law of Pharmaceutical Products, Medical Devices and Health Products; and is regulated by Supreme Decree No. 016-2011-SA and its amendments.
Likewise, the evaluation of the quality, safety and functionality of medical devices made by DIGEMID, takes into consideration the international standards proposed by the Global Harmonization Task Force (GHTF), currently denominated International Medical Device Regulators Forum (IMDRF), in accordance with the provisions in Article 55 of Law No. 29459. Among the requirements established, can be found a summary of the design verification and validation documents (items E and F). Design verification and validation are part of several stages involved in the design of a medical device, so in order to have a better understanding of them, it is necessary to know these other stages.
The design of a medical device is divided into stages, which are the following, according to established by IMDRF:
- Inputs
- Outputs
- Revision
- Verification
- Validation
- Transfer
- Change Control
These stages correlate with each other, in the different stages of medical device design development, see Figure 1.
A. Planning
First stage where the design of the medical device is defined, as well as the following aspects:
- Periodicity of product design revisions.
- Define responsibilities and authorities.
- Define verification, validation and transfer activities.
- Establish methods to ensure traceability of results.
- Establish the resources to carry out the activity.
B. Inputs
These are the requirements, which must be complied with and are applicable to the evaluated medical device for its proper performance. These requirements are composed of international (ISO, ASTM, etc.) or local (NTP) standards and are of the following types:
- Functional, suitability for use and safety requirements in accordance with the intended use.
- Regulatory requirements.
- Requirements derived from previous similar designs.
- Biocompatibility requirements, if applicable.
- Requirements applicable to risk management analysis, as established by the manufacturer.
- Other essential requirements related to the design and development of the product.
C. Outputs
These are the results obtained after verification and validation, which must comply with the input requirements. Likewise, these outputs must contain or refer to the product acceptance criteria and specify the characteristics of the product that are essential for its safe and correct use.
D. Revision
Through the revisions, the capability of the design outputs to meet the requirements is evaluated, and in case problems are identified, the necessary actions are proposed. The revisions are conducted at different stages and their periodicity of execution depends on what is established by the manufacturer.
E. Verification
This stage verifies that the product complies with the stipulated requirements, which means that of the input elements. This is achieved through product testing. Among the tests used are the following:
- Packaging integrity tests.
- Biocompatibility tests of materials.
- Sterility tests.
- Electromagnetic compatibility tests.
- Electrical safety tests.
- Risk analysis.
- Functional performance tests.
- Compliance with essential safety and efficacy requirements.
F. Validation
It is performed to ensure that the resulting product is capable of complying with the requirements for its intended application or purpose. Any process that cannot be verified is validated. Validation methods include:
- Clinical Evaluation Report
- Safety, sterilization, electromagnetic, packaging or other validation as applicable.
- Manufacturing process validation.
G. Transfer
It is performed once the registration of the product has been obtained. It is the step to the production stage of the final medical device.
H. Change control
Any change made at any stages of the product design. These changes must be reviewed, verified, validated and approved before implementation.
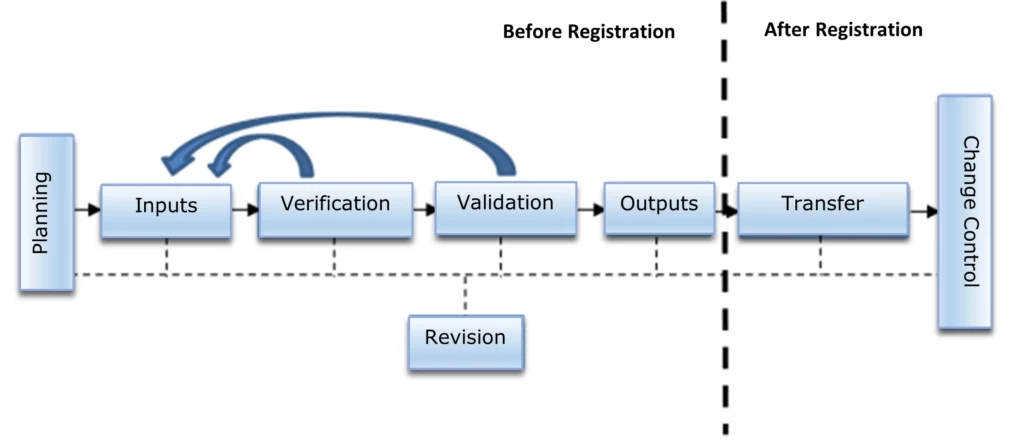
Figure 1: Diagram of the stages in the design of a medical device (Own authorship)
Although the processes at each stage of the development and design of a medical device are precisely known by the manufacturer, it is important that the regulatory authorities and pharmaceutical companies that are managing the sanitary registration of these products are able to know and interpret these stages. This in order to be able to carry out an objective evaluation and submission of the products that will enter the Peruvian market, since they will ultimately have an impact on the health of the general population.

