Regulatory Aspects to be taken into account for the Harmonization of Pharmaceutical Products Labeling in Bolivia and Peru
Q.F. Idaly Jazminy Morote Guzmán
QA Specialist & Biologics and Drug Regulatory Affairs Coordinator
SCR Consulting Peru
Q.F. Mary Carmen Chuquimia Socpaza
Quality Specialist & Regulatory Affairs Coordinator
SCR Consultores Bolivia
The Pan American Network for Drug Regulatory Harmonization (PANDRH), made up of the Drug Regulatory Authorities of 35 Member States and representatives of the Latin American Federation of the Pharmaceutical Industry (FIFARMA) and the Latin American Association of Pharmaceutical Industries (ALIFAR), as the regional associations of the Pharmaceutical Industry of the Americas, promotes the regional harmonization initiative since November 1997, with the First Pan American Conference on Drug Regulatory Harmonization, which was officially established in November 1999, during the Second Conference; the purpose is the search for common bases within the framework of recognized International Standards, taking into consideration the differences in the political, health and legislative realities of the countries in the Americas.
Bolivia, through the “Agencia Estatal de Medicamentos y Tecnologías en Salud” (AGEMED), as a member of the PANDRH, recognizes the Technical Document No. 10: Requirements for Medicines Registration in the Americas, and therefore adopts in part, the information on labeling in that document.
Peru, through D.S. No. 016-2011-SA and its amendments: Regulations for the Registration, Control and Sanitary Surveillance of Pharmaceutical Products, Medical Devices and Health Products establishes the requirements for the primary and secondary labeling of pharmaceutical specialties (chemically synthesized medicine), which are considered within the classification of Medicines and these in turn, of the classification of Pharmaceutical Products, while the present text when referring to Pharmaceutical Products for Peru, will be framed to the requirements of pharmaceutical products.
This article provides an overview of the requirements concerning the regulation of pharmaceutical products labelling in Peru and Bolivia, for their registration and commercialization, that allow harmonization, according to the Peruvian Regulation D.S. No. 016-2011-SA and its amendments: Regulation for the Registration, Control and Sanitary Surveillance of Pharmaceutical Products, Medical Devices and Health Products and the Bolivian Regulation with Law No. 1737: Law of Medicines and D.S. No. 25235: Regulation to the Law of Medicines.
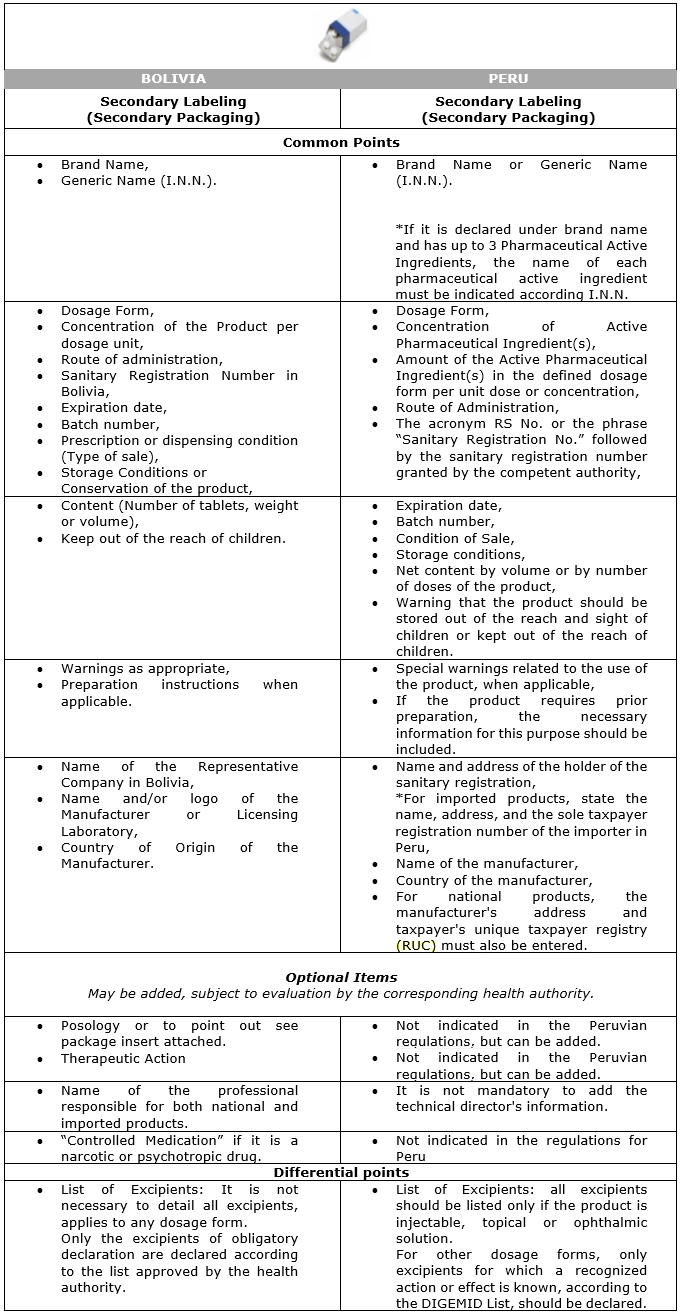
The following is a comparative table showing the information that must be included on the labels of pharmaceutical products in Peru and/or Bolivia that are sold with a medical prescription, according to the regulations in force in each country.
Table No. 01:
Information to be included in the Secondary Labeling of Pharmaceutical Products sold under Prescription:
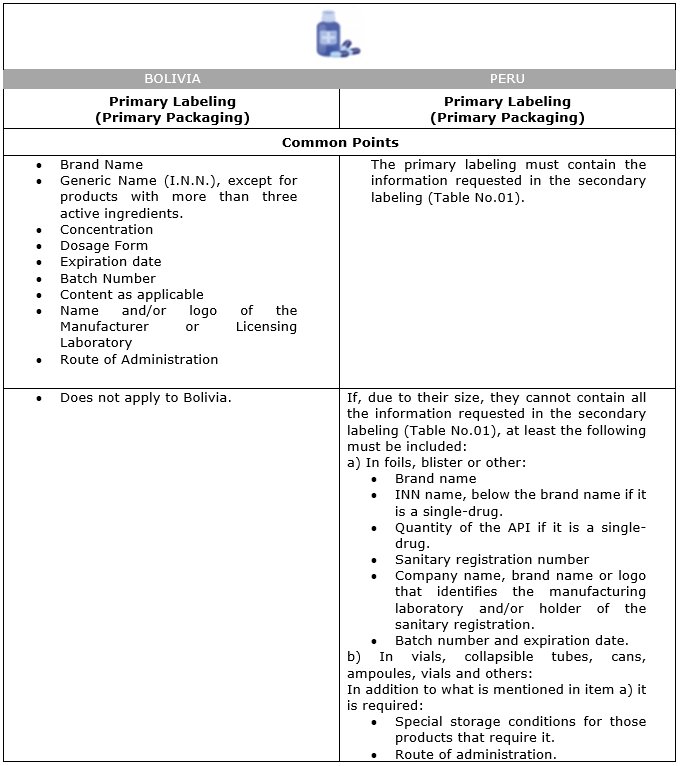
The information for primary labeling of pharmaceutical products sold with a Prescription must be as follows, according to the regulations in force in each country:
Table No. 02:
Information to be included in the Primary Labeling of Pharmaceutical Products to be sold under Prescription:
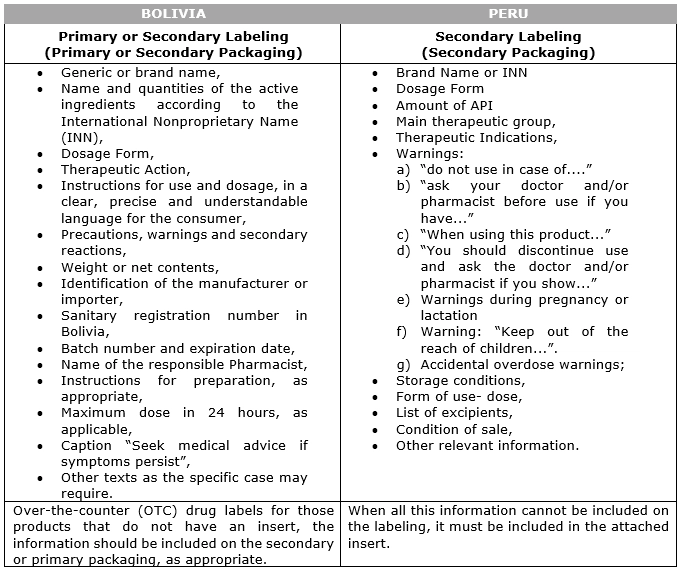
The following information must be included on the labeling of containers sold without a prescription in accordance with the regulations in force in each country:
Table No. 03:
Information to be included in the Labeling of pharmaceutical products whose condition of sale is without Prescription:
Below are some comparative considerations to be taken into account for labeling in each of these countries, according to R.M. No. 909: Manual for Sanitary Registration in Bolivia and D.S. No. 016-2011- SA and its amendments: Regulation for the Registration, Control and Sanitary Surveillance of Pharmaceutical Products, Medical Devices and Health Products, in Peru:
In general, AGEMED will accept labels or artworks, with legends in several languages as long as one of them is Spanish according to section 2.7.1. Item C of the Manual for Sanitary Registration. While the “Dirección de Medicamentos, Insumos y Drogas” (DIGEMID) accepts labels in Spanish language and additionally other languages can be added as long as they correspond to the information presented in the sanitary registration of the product, in line with the provisions of Article No. 17 of the Regulations for the Registration, Control and Sanitary Surveillance of Pharmaceutical Products, Medical Devices and Health Products.
In the case of imported products that due to the volume destined to Bolivia cannot include the Sanitary Registration number from the origin, it is accepted under the company’s responsibility, the use of a sticker or printed seal that includes the Number and year of the Sanitary Registration in Bolivia according to section 2.7.1. Item E of the Manual for Sanitary Registration. While the DIGEMID in Peru indicates that labels (stickers) may not be attached to correct or add information, except for importer’s data or any express indication by the authority, information may be added on the labeling whose printing (printed seal) must be clear, legible and indelible.
In Bolivia, for imported products, the legends authorized by the Drug Regulatory Authority of the country of origin will be accepted, as long as the difference with the national standard does not constitute a health risk according to section 2.7.1. Item H of the Sanitary Registration Manual. While the DIGEMID in Peru does not accept the inclusion of additional information that has not been previously approved or communicated.
From the aforementioned evaluation, it can be concluded that although there are divergent points between both authorities in Bolivia and Peru, there are points in common that companies can use to manage the same labeling for both countries and harmonize it according to the regulations, which would support better logistics in the distribution of pharmaceutical products

