Scope of Resolution N° 886: “Guide for Medicines Stability Studies in Bolivia”
Q.F. Mary Carmen Chuquimia Socpaza
Quality Specialist & Regulatory Affairs Coordinator
SCR Consulting Bolivia
The safety and efficacy of pharmaceutical products are influenced not only by their intrinsic properties, but also by their stability during the manufacturing process. Based on these premises, manufacturers and the Bolivian Drug Regulation Authority must work together to ensure that all pharmaceutical products put on the market undergo stability studies to establish their shelf life.
Let us remember that no product or its precursor elements are stable in a strict absolute sense, and we must seek to reliably ensure that each drug that reaches the patient is safe, effective and of acceptable purity, since its chemical identity, color, consistency, among other characteristics may change during the time elapsed from its manufacture to the time of its final consumption. These are characteristics that provide the consumer with the assurance that the drug is in acceptable conditions.
In this context, and in view of the advancement of knowledge and the growing harmonization that is taking place in the field of medicines, the need for the Bolivian Ministry of Health, through Agencia Estatal de Medicamentos y Tecnologías en Salud (AGEMED), to establish a Stability Study Guide for finished pharmaceutical products marketed in Bolivia has been exacerbated, thus further guaranteeing the quality of medicines. This Technical Document titled: “Guide for Drug Stability Studies” was approved on October 15, 2020, through
Administrative Resolution N° 886, which is conformed of five chapters and seven annexes.
This guide is a complement to the current regulations in Bolivia; therefore, the stability of pharmaceutical products is carried out in accordance with the Drug Law N° 1737, Good Manufacturing Practices Standards, Good Manufacturing Practices Inspection Guide and other related regulations in force.
This Guide on Drug Stability Studies has been structured taking as a reference the documents published by the WHO in relation to the requirements for the development and presentation of stability studies, for different purposes, from the Sanitary Registration process to the verification of GMP compliance, such as Report 29, Report 34 Annex 5 “Guidelines for stability evaluation”, Report 43 Annex 2 “Stability tests for API and finished product”, Report 52 Annex 10 “Guidelines for stability tests of active pharmaceutical ingredients and finished pharmaceutical products”; those published by ICH guideline “Q1A-R Stability test of new pharmaceutical substances and products” referring to the requirements for the development and presentation of stability studies; Secretarial Resolution N° 0296: Good Manufacturing Practices Standard and Ministerial Resolution N° 0909: Manual for Sanitary Registration of Medicines.
The objective of this Technical Document is to establish the requirements that must be fulfilled in the presentation of stability studies of finished pharmaceutical products, to obtain the information that allows to propose the shelf life of the drug (validation period) and to establish the storage conditions to guarantee quality, safe and effective medicines in the application for their sanitary registration. Considering that the same Guide is applicable to new Finished Pharmaceutical Products (FPP) and to those already marketed in the Bolivian national territory, in the development of stability studies under environmental conditions at a temperature of 30ºC ± 2ºC and Relative Humidity of 75% RH ± 5%, which correspond to the indicated environmental conditions for the climatic zone IVb, established by the World Health Organization-WHO, for Bolivia; and is excluded according to the guidelines, for the development of stability studies of biological and biotechnological products.
AGEMED, as the regulatory agency, establishes the guidelines and regulations regarding the performance of stability studies, and it is the responsibility of the manufacturer to comply with the Authority’s provisions and the Authority to verify such compliance.
For this reason, the Guide of Drug Stability Studies recommends techniques for conducting stability studies, in order to facilitate both their performance and evaluation to assign the shelf life to finished pharmaceutical products, and to ensure unalterable potency, identity, quality and purity, from their preparation and during their entire period of efficacy; taking into account that it is the manufacturer’s responsibility, as part of the development of a pharmaceutical product, to design and carry out adequate stability studies, allowing to obtain reliable information and demonstrating how its quality varies over time and under the influence of the storage conditions to which it is submitted. This information will allow him to propose the period of validity, during which the product can be used with absolute safety.
Likewise, the manufacturer is responsible that the finished pharmaceutical products maintain their quality during their permanence in the market, as long as they are distributed, handled, stored or dispensed by third parties under the conditions required in the declared packaging and storage.
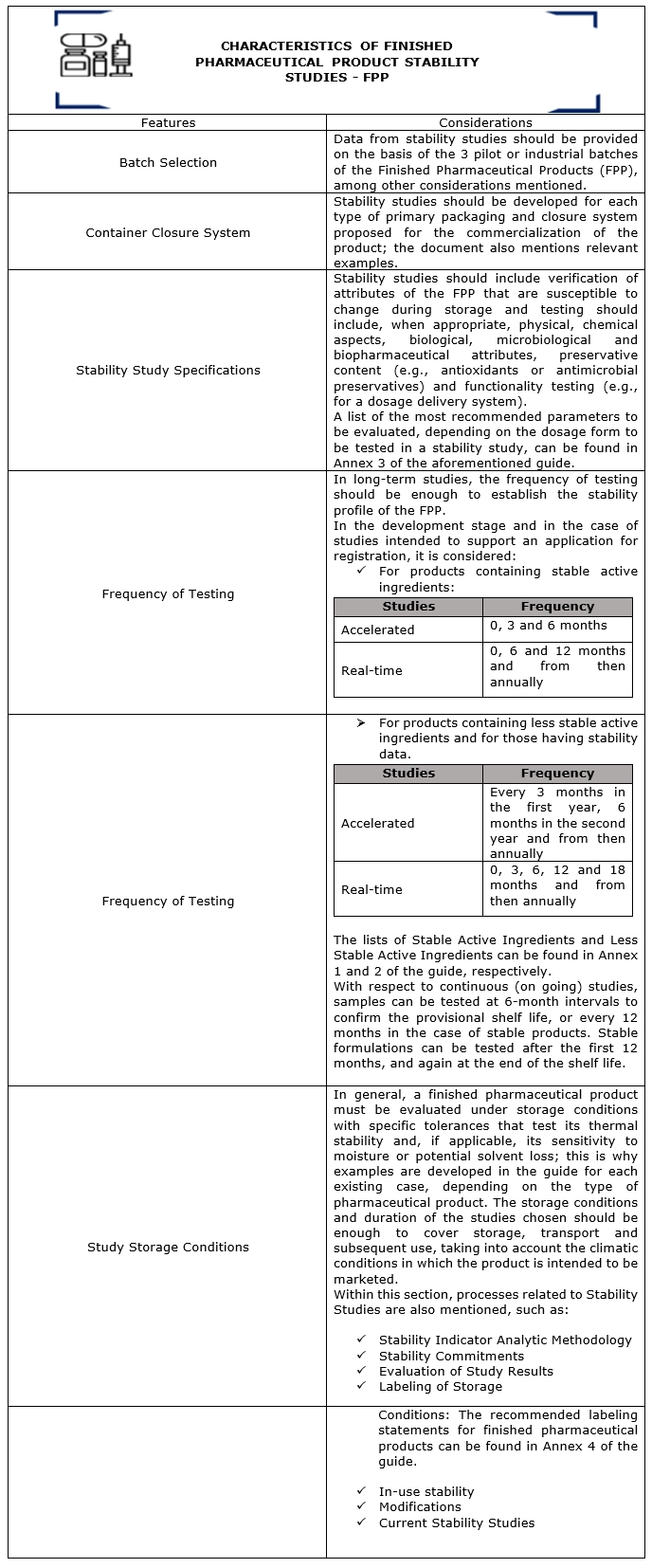
In chapter II of this guide explains the characteristics that the Stability Studies of Finished Pharmaceutical Products must comply with and in which, in brief, some considerations are provided:
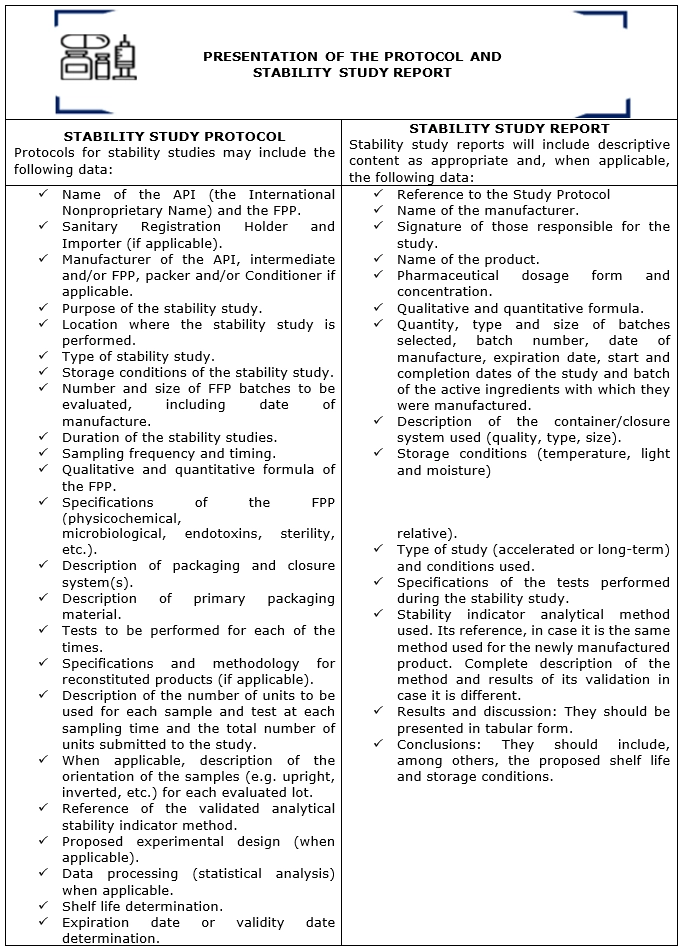
 Chapter III contains the necessary guidelines for the presentation of the stability study protocol and report, recommending that the stability study protocol and report include the following information and data to facilitate decisions related to the acceptance of the stability study and the proposed shelf life:
Chapter III contains the necessary guidelines for the presentation of the stability study protocol and report, recommending that the stability study protocol and report include the following information and data to facilitate decisions related to the acceptance of the stability study and the proposed shelf life:
In addition, Chapter IV explains the tentative validity period, indicating that when accelerated studies for 6 months do not show significant changes in the chemical or physical specifications and microbiological stability evaluated (beginning and ending) or long-term studies for 6 months do not show variability (beginning and 6 months), a maximum validity period of 24 months (2 years) will be granted, with the support of the study.
In case the product is temperature sensitive (FPPs to be stored under special conditions), only long-term stability studies for a minimum of 6 months may be submitted, with a maximum validity period of 24 months.
Finally, the seven annexes of the normative document are as follows:
- ANNEX Nº 1 List of Stable Active Ingredients.
- ANNEX Nº 2 List of Less Stable Active Ingredients.
- ANNEX Nº 3 Recommended parameters that can be studied in each dosage form for finished pharmaceutical products.
- ANNEX Nº 4 Recommended labeling statements for finished pharmaceutical products.
- ANNEX Nº 5 Gradualness for compliance with stability studies for national pharmaceutical products.
- ANNEX Nº 6 Classification of finished pharmaceutical products based on high and intermediate health risk.
- ANNEX Nº 7 Finished pharmaceutical products to be stored under special conditions.

