Evaluation of Safety and Efficacy Documents for the Sanitary Registration of Medicines in Bolivia
Q.F. Mary Carmen Chuquimia Socpaza
Quality Specialist & Regulatory Affairs Coordinator
SCR Consulting Bolivia
The process of evaluation and granting of the Sanitary Registration of Medicines in Bolivia is responsibility of the “Agencia Estatal de Medicamentos y Tecnología en Salud” of the Ministry of Health and Sports, through the Department of Marketing Authorization, specifically through the Medicines Evaluation Area and the National Pharmacological Commission, as a technical advisory body on the evaluation of Efficacy and Safety. Its main purpose is to pharmacologically select the medicines that could be marketed in the country, evaluate their indications and contraindications under technical and scientific criteria, with the purpose of guaranteeing their efficacy and their rational use by the population, this process is inevitably and strictly based on both international and national clinical trial standards, in line with the continuous progress of Evidence-Based Medicine.
The National Pharmacological Commission must be composed by members of different institutions, who must have specific merits in the in the management of medicines, extensive pharmacological expertise, recognized professional practice, unobserved ethical conduct, commercial and/or labor independence with national or foreign pharmaceutical industrial laboratories, as well as with marketers and NGO’s with activity dedicated to the health area, according to what is established by the Regulation of the National Pharmacological Commission a Ministerial Resolution No. 0138 of April 14, 1998.
Therefore, any substance considered as a medicine in Bolivia, in order to proceed with the Sanitary Registration, must previously have the pharmacological evaluation by the National Pharmacological Commission, having to present the forms according to the case (form 007 and/or form 019) and the pharmacological monograph including preclinical and clinical data of the product according to the specifications detailed in Annex No. 9 Pharmacological Information of the Sanitary Registration Manual approved through Ministerial Resolution No. 0909 of December 7, 2005.
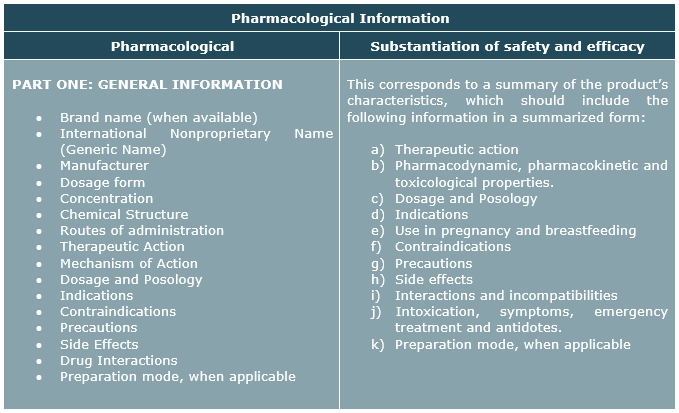


Regardless of the submission of Form 007, for products involving a new molecule (new chemical entity), which refers to the active ingredient not previously registered in Bolivia, or with new indications, new associations or products that due to recent clinical studies have alerts, the Efficacy and Safety Evaluation Form for New Medicines Form 019, Annex No. 8 of the Sanitary Registration Manual, including the evaluation report filled out by the evaluators, must be attached to the dossier.
The evaluation by the National Pharmacological Commission (CFN) is subject to chronological order, who, reviewing the requirements presented, will proceed to the evaluation and qualification of Efficacy and Safety of the medicine and according to its regulations will issue the corresponding decision, which may be:
a) Approved: In case the product is approved, the interested party may request at the Evaluation and Registration window the signed Qualification Request – Form 007, which constitutes an endorsement to continue with the Sanitary Registration process according to the provisions of the Sanitary Registration manual.
b) Observed: In case the product is observed or presents a determination of critical evaluation of efficacy and safety for new molecule or when the CFN so determines, the interested party may request at the Evaluation and Registration window, the Qualification Request Form – Form 007 submitted, signed with the justification of the observation.
The interested party must correct the observation or submit the complementary information requested by the Commission, within a term not to exceed 90 days, attaching a new Qualification Request Form. Once this term has elapsed and the requested information has been complied with, the Commission will approve or reject the product. If the interested party does not present the documentation within the term indicated, the process will be archived without right to claim.
c) Rejected: In case the product is rejected, the interested party will have to request at the Evaluation and Registration window the signed Request for Qualification Form 007, with the basis for rejection. The interested party may request an appeal with scientific grounds and in case of ratification of the decision, the “Agencia Estatal de Medicamentos y Tecnología en Salud” will not grant the Registration under any circumstances.
A rejected product may not be requalified by the National Pharmacological Commission until one year after its rejection.
The evaluation time by the National Pharmacological Commission takes about 6 months to a year.
The National Pharmacological Commission, through the “Agencia Estatal de Medicamentos y Tecnología en Salud”, publishes a periodic quarterly update, on the Website, the list of the active ingredients and associations evaluated, qualified and approved by the same.
It should be noted that the “Agencia Estatal de Medicamentos y Tecnología en Salud” can directly evaluate the applications for Sanitary Registration submitted with the corresponding Qualification Request Form – Form 007, when the drug is included in the current National List of Essential Medicines or refers to an endorsement of the National Pharmacological Commission that has one of the following similarities:
- Similarity in dosage form, active ingredient(s) and different concentration, as long as the concentration requested is within the margins indicated in pharmacopoeia recognized by Law.
- Similarity in active ingredient(s), concentration, different dosage form but same route of administration.
It is also important to point out that at the moment of using a reference endorsement, the product to be registered must have similarity regarding certain information that is registered in the endorsement, such as therapeutic action, indications and dosage, in that sense the regulatory requirements for the Sanitary Registration process, such as the leaflet and the pharmacological monograph must be adapted to what is described in the reference endorsement, otherwise, all the documentation described above must be submitted for the National Pharmacological Commission to approve and issue a new endorsement according to the product to be registered.

